Question: What mechanisms regulate ketogenesis and moderate its impact on disease? Which conditions are directly impacted by ketones and/or a ketogenic diet?
Takeaway: Ketogenesis is regulated, directly and indirectly, by the action of insulin. The ketogenic diet has clear clinical benefits for certain rare conditions involving defects in glycolytic pathways. Preliminary evidence also indicates it may be of benefit for patients with cancer, neurodegenerative disease, and various conditions related to inflammation.
This 2019 review summarizes the major mechanisms governing ketogenesis and analyzes the effects of dietary ketosis on specific disease states.
Ketogenesis — that is, synthesis of ketone bodies in the liver — is primarily brought on by an extended suppression of insulin levels (1). During the initial hours of a fast, or during initiation of a very low-carbohydrate diet, glucose stores in the liver and muscles will be released into the blood to maintain blood glucose levels. Once these stores are exhausted, fatty acids are released from the adipose tissue. When these fatty acids reach the liver, they undergo beta oxidation and form acetyl-CoA. When acetyl-CoA levels become sufficiently high, the liver begins producing ketone bodies (KBs) in significant quantities (2). These circulating ketone bodies are then taken up by other cells and organs via monocarboxylate transporter 1 (MCT1), where they are then converted back into acetyl-CoA to be used as an energy source (3).
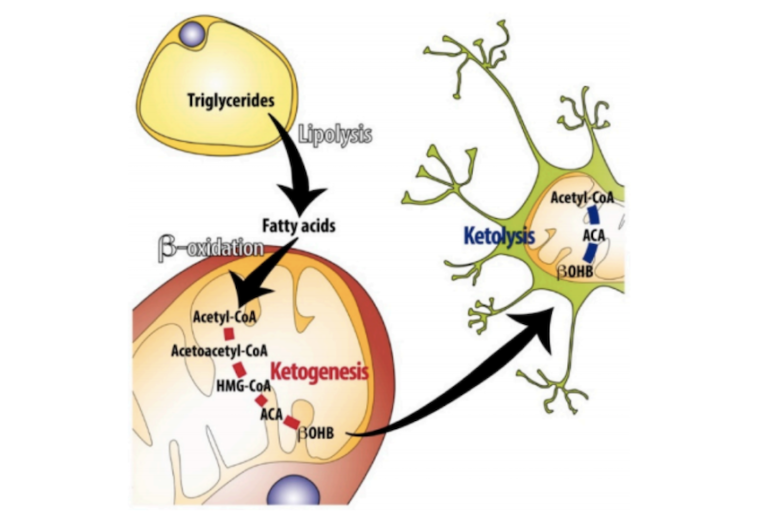
Figure 1: Summary of the key processes regulating ketogenesis
Ketogenesis is thus primarily regulated by the availability of fatty acids to the liver. By this mechanism alone, ketogenesis is suppressed by even a modest elevation of insulin levels, which shifts fat cells from fatty acid breakdown and release toward fatty acid storage, reducing the flux of fatty acids into liver cells (4). Insulin simultaneously suppresses ketosis through direct regulatory effects on liver cells, including the elevation of malonyl-CoA, which reduces the availability of a key ketogenic intermediate (acyl-CoA) within hepatic mitochondria, thus inhibiting ketogenesis (5).
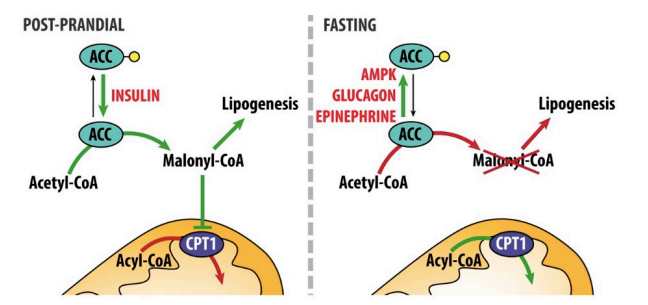
Figure 2: Regulation of ketogenesis by insulin. Red arrows indicate processes that are downregulated or turned off in each circumstance. Insulin, via its effects on malonyl-CoA production, suppresses acyl-CoA availability in hepatic mitochondria, which in turn suppresses ketogenesis.
The ketogenic diet (KD) — that is, a diet that induces ketosis, generally through dramatic suppression of carbohydrate intake — has clear and specific clinical utility for two conditions, both characterized by defects in glycolytic pathways. GLUT1 deficiency syndrome (GLUT1 DS) impairs glucose transport across the blood-brain barrier and leads to low levels of glucose in the cerebrospinal fluid. Symptoms include seizure, movement disorders, and cognitive dysfunction. The ketogenic diet rapidly resolves these symptoms; it is recommended patients with this genetic condition initiate a KD as soon as possible after diagnosis to prevent long-term brain damage (6). Pyruvate-dehydrogenase deficiency syndrome (PDH-DS) is similar in that a ketogenic diet will ameliorate symptoms by circumventing pathologies related to central glycolytic pathways (7). The well-documented benefits of a ketogenic diet in patients with drug-resistant seizures may occur through similar mechanisms (8), though in these cases a KD is generally maintained alongside antiepileptic drugs.
In these and similar conditions, the KD shifts cellular metabolism away from glycolysis. On a KD, 90% of energy is derived from fat and only 10% from carbohydrate and protein, reducing both the need and potential for glycolysis. Seizures, for example, are characterized by a high glycolytic rate; the ketogenic diet suppresses glycolysis and thus reduces seizure risk (9). KDs also may have more direct, secondary benefits on these conditions by preserving neuronal energy homeostasis and mitochondrial health. Low neuronal ATP levels increase neuronal excitability via the effects of (a lack of) ATP on potassium channels (10); ketogenic diets support increased cellular ATP levels, particularly in the context of glycolytic dysfunction. KDs simultaneously reduce production of reactive oxygen species (ROS) by cellular mitochondria and promote mitochondrial biogenesis within neurons (11). These and other effects on specific molecules like adenosine and individual fatty acids may further explain the benefits of a ketogenic diet on seizure prevention and control (12).
In the periphery, ketone bodies are anti-inflammatory due to their inhibition of NLRP3 (13). Animal studies have provided preliminary evidence indicating a ketogenic diet can prevent or reduce severity of conditions directly tied to inflammation, like gout and arthritis (14). These same effects have led to the suggestion that a ketogenic diet can manage the chronic, low-grade inflammatory state associated with cardiovascular disease, thus reducing heart disease risk (15).
The role of the ketogenic diet in cancer treatment and prevention has been discussed extensively on CrossFit.com (16). In theory, a ketogenic diet exploits the Warburg effect, depriving glycolysis-dependent cancer cells of a primary fuel source without harming healthy cells. At least some cancer cells, however, are able to use ketone bodies as fuel, nullifying this effect. Nevertheless, current evidence indicates a KD consistently improves quality of life in cancer patients and in particular reduces the gastrointestinal and neurological side effects of existing cancer therapies (17). The use of a ketogenic diet as an adjuvant to chemotherapy, radiotherapy, and other cancer treatments will continue to be an area of exploration.
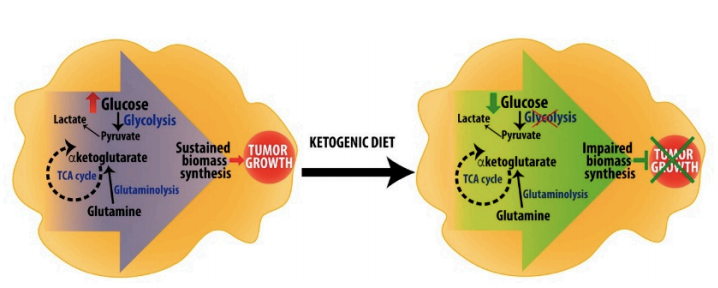
Figure 3: Hypothetical mechanisms by which a ketogenic diet impairs tumor growth
Early evidence suggests a ketogenic diet (or artificial elevation of serum ketones by use of ketone supplements) may temporarily restore cognitive function in patients in the early stages of neurodegenerative disease (18). The authors of this review argue at least some cases of Alzheimer’s disease are metabolic in nature, so a ketogenic diet, by supporting long-term maintenance of neuronal energy homeostasis, may prevent and even reverse neurodegeneration. However, there is little data to support this theory. Similarly, preliminary evidence suggests a ketogenic diet may improve non-motor symptoms in patients with Parkinson’s disease (19). Given that these symptoms are poorly addressed by the current standard of care (L-DOPA), the KD is viewed as a promising adjuvant therapy for patients with Parkinson’s.
The KD has also been proposed as a treatment for fatty liver disease, as the carbohydrate restriction associated with a KD leads to rapid clearance of liver fat stores (20). Notably, use of a KD to treat NAFLD may lead to a virtuous cycle, as a liver free of fat is able to more quickly initiate ketosis in response to insulin suppression than a fattier liver.
Minimal data, mostly drawn from children, has suggested long-term adherence to a KD may be associated with increased risk of fractures, kidney stones, and delayed growth (21). However, this same evidence suggests these side effects may be the result of poorly constructed ketogenic diets that lead to vitamin D deficiency and similar conditions and may not be inherent in the diet itself.
Overall, this review provides evidence that supports the use of a KD in a variety of inflammatory conditions, cancers, and neurological disorders. Research studying the ketogenic diet remains limited, but the KD remains a promising option for conditions in which defects in glycolysis, cellular energy homeostasis, or inflammation play a role.
Notes
- Hepatic ketogenic insufficiency reprograms hepatic glycogen metabolism and the lipidome; Regulation of ketone body metabolism and the role of PPARa
- Lehninger Principles of Biochemistry
- Ketone bodies as signaling metabolites
- Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry
- Ibid.
- Effects of the ketogenic diet in the glucose transporter 1 deficiency syndrome; Introduction of a ketogenic diet in young infants; A mouse model for Glut-1 hapolinsufficiency
- Efficacy of ketogenic diet for pyruvate dehydrogenase complex deficiency
- Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets: Studies in patients with identical mutations; Ketogenic diets for drug-resistant epilepsy; Modified ketogenic diets in adults with refractory epilepsy: Efficacious improvements in seizure frequency, seizure severity, and quality of life; How often is antiseizure drug-free ketogenic diet therapy achieved?
- Practice Paper of the Academy of Nutrition and Dietetics: Classic and Modified Ketogenic Diets for Treatment of Epilepsy; Brain energy metabolism during experimental neonatal seizures
- Mitochondria and neuronal activity
- Modulation of oxidative stress and mitochondrial function by the ketogenic diet; Role of oxidative stress in epileptic seizures; Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet
- Ketogenic diet suppresses seizures in mice through adenosine A (1) receptors; Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet; Dose-dependent anticonvulsant effects of linoleic and a-linolenic polyunsaturated fatty acids on pentylenetetrazol induced seizures in rats
- The ketone metabolite B-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease
- B-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares
- Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: Is low-grade inflammation the neglected component?
- Tumor metabolism, the ketogenic diet and B-hydroxybutyrate: Novel approaches to adjuvant brain tumor therapy; The ketogenic diet for the treatment of malignant glioma; Treatment of malignant gliomas with ketogenic or caloric restricted diets: A systematic review of preclinical and early clinical studies; A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth; Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake; Serial MRI imaging reveals minimal impact of ketogenic diet on established liver tumor growth; Anti-tumor effects of ketogenic diets in mice: A meta-analysis; The emerging role of ketogenic diets in cancer treatment
- Beneficial effects of ketogenic diets for cancer patients: A realist review with a focus on evidence and confirmation; A ketogenic diet reduces central obesity and serum insulin in women with ovarian or endometrial cancer; Favorable effects of a ketogenic diet on physical function, perceived energy, and food cravings in women with ovarian or endometrial cancer: A randomized, controlled trial; The potential use of a ketogenic diet in pancreatobiliary cancer patients after pancreatectomy
- Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease); Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease; Changes in regional cerebral blood flow associated with a 45-day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease; Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial; The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease
- Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study; Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial; How can a ketogenic diet improve motor function?
- Multi-dimensional roles of ketone bodies in fuel metabolism, signaling and therapeutics; Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia; Short-term weight loss and hepatic triglyceride reduction: Evidence of a metabolic advantage with dietary carbohydrate restriction
- Complications during ketogenic diet initiation: Prevalence, treatment and influence on seizure outcomes; Impact of the ketogenic diet on linear growth in children: A single-center, retrospective analysis of 34 cases; Ten-year, single-center experience of the ketogenic diet: Factors influencing efficacy, tolerability and compliance; The MCT-ketogenic diet as a treatment option in refractory childhood epilepsy: A prospective study with 2-year follow-up; Long-term outcome and tolerability of the ketogenic diet in drug-resistant childhood epilepsy – The Austrian experience; The effect of the ketogenic diet on the developing skeleton; Use of potassium citrate reduces kidney stone incidence with the ketogenic diet
Ketogenic Diet: Shining a Light on Old But Gold Biochemistry